Establishing Effective Medication Risk-Reduction Strategies Everywhere Around the World
Specific medication safety issues are well known to cause harmful and fatal errors in patients despite knowledge of repeated occurrence and warnings. These deadly events have the following common characteristics (1):
- They are recurring, likely to happen to another patient if not addressed
- They are identifiable, easily recognized, clearly defined and not attributable to other possible causes
- They are avoidable, by appropriate practices, measures and organizational barriers
Preventing these deadly events is possible by the implementation of risk-reduction strategies that reduce or eliminate the possibility of errors, make errors visible, and minimize their consequences (2). The primary goal is to redesign the medication management process to make it harder for errors to reach the patient. The fact that such deadly adverse events can be prevented by using specific measures and organizational checks and balances has led numerous facilities to call them “never events.” In this expression, “never event” is a clear call-to-action, rather than an expectation of perfect performance or an attempt to blame if such an event happens. For this call-to-action, the International Medication Safety Network (IMSN) has identified four risk-reduction strategies, herein called the Global Targeted Medication Safety Best Practices, to inspire and mobilize widespread, international adoption of consensus-based best practices for specific medication safety issues that continue to lead to harmful and deadly medication errors.
The first four IMSN Global Targeted Medication Safety Best Practices are:
- Global Medication Safety Best Practice 1
Remove potassium concentrate injection from drug storage areas on all inpatient nursing units/wards - Global Medication Safety Best Practice 2
Prepare and dispense vinca alkaloids in a minibag, never in a syringe - Global Medication Safety Best Practice 3
Prevent inadvertent daily dosing of oral methotrexate for non-oncologic conditions - Global Medication Safety Best Practice 4
Prevent errors related to improper preparation of 2-component vaccines
Focusing on these four high-alert medications—potassium concentrate injection, vinca alkaloids, oral methotrexate used in non-oncologic conditions, and 2-component vaccines—is emblematic of the changes in systems and practices that should be undertaken as a priority around the world. High-alert medications bear a heighted risk of causing patient harm when used in error.
While Best Practices 1 and 2 target the acute care setting, serious adverse events with potassium concentrate injection and vinca alkaloids have occurred in other settings, such as ambulatory practices and ambulatory procedure areas. Best Practice 3, associated with oral methotrexate, applies to all settings, including long-term care and home care.
These Global Targeted Medication Safety Best Practices have been reviewed and endorsed by experts from the International Medication Safety Network, an association of medication safety organizations, pharmacovigilance centers, regulatory agencies and medication safety experts. They have already been successfully adopted by numerous organizations (3). For example, in the United States, the Institute for Safe Medication Practices (ISMP) has documented a steady progression between 2014 and 2017 of implementation of the Targeted Medication Safety Best Practices for Hospitals associated with two of the four targeted high-alert medications, vinca alkaloids and oral methotrexate.
Risk-Reduction Strategies for Preventing Deadly Medication Errors
Selecting the best error-reduction strategy is not easy (4). As illustrated below, risk-reduction strategies tend to focus on system design, which are often most effective, and/or human factors principles, which are less effective that system design strategies (4-7) .
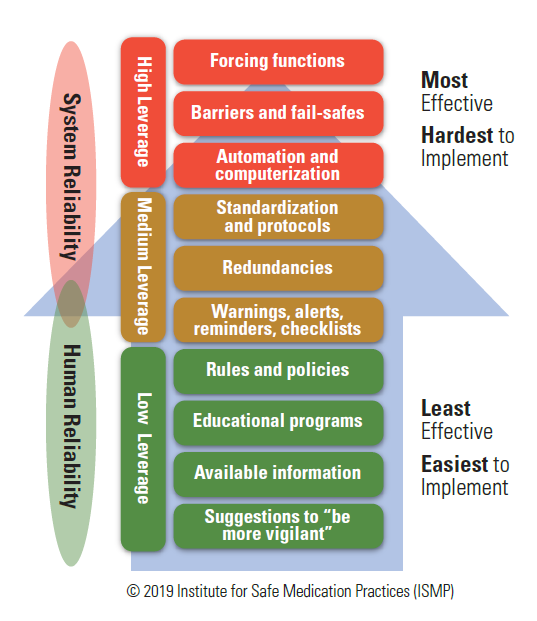
- High-leverage strategies that focus on the system and ‘design out’ hazards are most effective because they can eliminate the risk of errors and associated harm. They do not rely heavily on individual human attention and vigilance. Such strategies include forcing functions, barriers and fail-safes, constraints, and automation and computerization. These strategies may involve complex implementation plans because they often require system redesign.
- Medium-leverage strategies do not eliminate hazards but reduce the likelihood of errors or minimize harm. They are relatively easy to implement but may need periodic updating and reinforcement to maintain knowledge and the currency of the process or product. These strategies are highly dependent on the behavior of people using the system. They include standardization; redundancies (e.g., independent double checks); reminders and checklists; warnings, alerts, and alarms; and patient counselling.
- Low-leverage strategies are often easy and quick to implement but need constant updating and reinforcement to maintain knowledge and currency. They aim to improve human performance and are more effective when combined with other medium- or highleverage strategies. Low-leverage strategies include rules, policies, procedures, guidelines, protocols, education and training, and information documents. Suggestions to “be more vigilant” have little value in reducing the risk of errors.
Acknowledgments and Disclosures. All reviewers were volunteers and received no compensation for their contribution to this work. IMSN is grateful for these volunteers and acknowledges their expertise and assistance in revising these Global Targeted Medication Safety Best Practices.
William Allan, Medication Safety Specialist, Health Quality and Safety Commission, New Zealand; Michael Cohen, Chair of IMSN, President of the Institute for Safe Medication Practices (ISMP), United States; Helen Dowling, Pharmacist Advisor, eHealth and Medication Safety, Australian Commission on Safety and Quality in Health Care (ACSQHC); BarbraKaryne Nchinda Fobi, International Medication Safety Fellow, ISMP, United States; Michael Hamilton, Physician Lead and Medication Safety Specialist, Institute for Safe Medication Practices Canada (ISMP Canada); Ciara Kirke, Health Service Executive Quality Improvement Division, Ireland; Pia Knudsen, Pharmacist, Senior Patient Safety Officer, Danish Patient Safety Authority; Ursula Köberle, Pharmacovigilance analyst, Drug Commission of the German Medical Association; Christina Michalek, Medication Safety Specialist, ISMP, United States; Maria Jose Otéro Lopez, President ISMP Spain; Gregory A. Poff, Chairman, Saudi Medication Safety Center, Saudi Food and Drug Authority; Stephen Routledge, Patient Safety Improvement Lead, Canadian Patient Safety Institute; Étienne Schmitt, Head of Programme Éviter l’Évitable (Preventing the Preventable), Prescrire, France; Diana Shipp, Senior Project Manager, eHealth and Medication Safety, ACSQHC; David U, General Secretary of IMSN, Medication Safety Advisor, ISMP Canada.
References:
- The Canadian Patient Safety Institute (CPSI). Never Events for Hospital Care in Canada - Safer Care for Patients September 2015; 11 pages. Download
- Cohen MR, Smetzer JL, Tuohy NR and Kilo CM. High-Alert Medications: safeguarding against errors. In Medication Errors. 2nd ed. Washington (DC): American Pharmaceutical Association. 2007; 317-411.
- Institute for Safe Medication Practices (ISMP). 2018-2019 Targeted Medication Safety Best Practices for Hospitals Access
- Institute for Safe Medication Practices (ISMP). Medication error prevention “toolbox”. ISMP Medication Safety Alert! June 2, 1999; 4 (11): 1.
- Institute for Safe Medication Practices (ISMP). Your high-alert medication list—relatively useless without associated risk-reduction strategies. ISMP Medication Safety Alert! 2013; 18 (8): 1-5.
- Institute for Safe Medication Practices (ISMP Canada). Designing effective recommendations. Ontario Critical Incident Learning: Improving Quality in patient safety 2013; (4): 1-2. Download
- Health Quality & Safety Commission New Zealand. Error prevention strategies. Medication Safety Watch February 2015; (13): 1-3. Download
Download the IMSN Global Targeted Medication Safety Best Practices - June 2019
