Prevent errors related to improper preparation of 2-component vaccines
Background
Several vaccines require mixing two components that are supplied by the manufacturer in physically separate containers. For example, a lyophilized powder and a vaccine-specific liquid diluent. Other vaccines may include a powder vial plus a liquid antigen vial or adjuvant component. These powder and liquid containers may be packaged together by the manufacturer or, in some cases, may arrive separately. Preparation of the vaccine for injection must be completed prior to administering the dose.
Unfortunately, errors sometimes occur when proper mixing does not happen and only one component of the two-component vaccine is administered, thus the patient will not be fully immunized (1). In other cases, an incorrect diluent is used to prepare the lyophilized powder, which may affect stability. Such errors may go undetected thus revaccination does not occur, potentially allowing the development of disease. For example, with rabies vaccine where only the diluent is given in error. Otherwise, errors may lead to a failure to protect the patient.
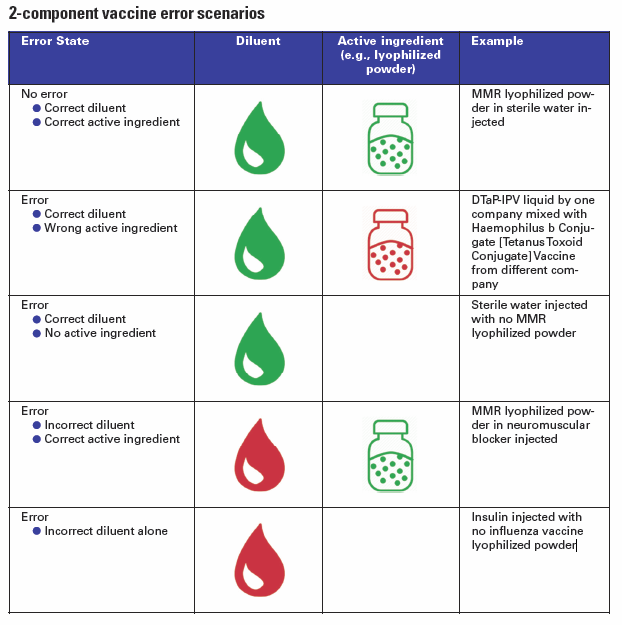
In some very dangerous cases, a dangerous substance was accidentally used in place of the vaccine diluent. For example, there have been several incidents where a paralyzing agent (neuromuscular blocker) has been used instead of a liquid diluent. In 2014 in Syria, fifteen children died after atracurium, a neuromuscular blocking agent, that was used to reconstitute a measles vaccine (2). More recently, in Samoa in 2018, two infants died after also receiving atracurium mixed with their measles, mumps and rubella vaccine. The nurses were charged and convicted for homicide and sentenced to jail terms (3).
Improper administration of vaccines is a public health concern. Negative publicity surrounding harmful vaccination-related errors has led to intense media coverage of incidents. In turn, this has increased anti-vaccine advocacy, which can result in a loss of public confidence. In the Samoan tragedy, routine measles immunization rates fell to just 31%, which contributed to a major measles outbreak when parents became afraid to have their children vaccinated. In the wake of the incident, the Samoan Ministry of Health reported over 4,300 cases of measles, including 70 deaths, 61 of which were in children under 5 years of age (4).
In summary, such errors can lead to lack of patient protection from preventable disease, inconvenience to patients and families for required revaccination if an omission is recognized, increased associated healthcare costs, and even a potential for adverse drug events (in some cases death), leading to criminal charges against healthcare providers.
Goal
The goal of this best practice is to prevent errors related to administration of the manufacturer-supplied diluent or liquid component by itself or use of an inappropriate diluent (different from the one provided by the manufacturers).
Best Practice Description
IMSN strongly advocates for high-leverage strategies to reduce the risk of errors with two-component vaccines and recommends healthcare practitioners, clinics, medical offices and institutions to:
- Circle or highlight critical information such as mixing instructions prominently on vaccine container labels. Also, use flag-type labels to remind users of the need to use the supplied diluent or, in cases where a diluent is not provided by the manufacturer, only the appropriate diluent as mentioned in product labeling.
- Dangerous drugs, such as paralyzing agents (neuromuscular blockers), insulin, and others must not be located nearby or stored in an area where vaccines are prepared and administered.
- Where the proper diluent is not provided by the manufacturer, managers should carefully examine supply chain issues that might possibly allow purchase and distribution of dangerous drugs to areas where vaccinations are prepared and administered.
- Clearly label or distinguish each component if the manufacturer’s label could mislead staff into believing either is the vaccine itself. Whenever available, use barcode scanning systems to ensure the correct components are utilized. Scanning of both components during preparation could help identify an incorrect vial or an inadvertent omission if only one vial is used inadvertently.
- Establish a process to keep two-component vaccines together if storage requirements do not differ, for example where a vaccine component must be frozen, but the diluent kept at room or refrigerated temperature). Dispense the products together in a bag with an auxiliary label to remind staff to use both vials.
- Document product identification number, lot number and expiration date of each vial in the vaccination record or log prior to administration to confirm appropriate selection or preparation of both components of two-component vaccines. (With electronic inpatient records, prompts should require documentation of both components of two-component vaccines.) Documenting actual administration of the vaccine should always occur immediately after the vaccine is administered.
- If possible, in doctor offices, clinics, pharmacies, etc., incorporate the patient or family member, such as a parent, into the checking process. Prepare them in advance for what will be administered and make them part of the checking process. This may not be possible during large scale vaccination campaigns.
- Be sure staff understand the differences between two-component vaccines, each with antigen, and vaccines requiring specific diluents or adjuvant liquids.
- Label the areas where vaccines are stored to facilitate correct selection and to remind staff to combine the contents of vials.
Rationale
These selected risk-reduction strategies include constraints that can help avoid errors related to two-component vaccines, such as providing premixed formulations if feasible, or integration of vaccines and liquid components in a single container, such as a dual chambered vial or syringe with powder in one chamber and liquid in the other. Upon breaking a barrier between the two chambers of a vial, the two components will mix prior to withdrawal for administration; a two chambers pre-filled syringe, without any withdrawal step appears as the best choice. For oral rotavirus vaccine, different formulations exist such that dilution of the vaccine powder is necessary in some countries while the same vaccine is available in a premixed formulation in others. Only premixed formulations should be marketed to avoid the dilution step altogether in order to provide the same safety level all around the world.
The risk-reduction strategies also include high-leverage forcing functions and computerization, such as never storing or distributing dangerous drugs to areas where vaccinations are stored, prepared and administered; and providing checking capabilities with barcoding systems in electronic systems and electronic hard-stop verification of the identity of the components and scheduling of the vaccine.
Improving 2-component Vaccines Packaging and Labelling Safety
In December 2015, the International Medication Safety Network (IMSN) issued a position statement that calls for greater worldwide attention to the problem of unsafe design of vaccine packaging and labelling. The statement called on pharmaceutical companies, technology vendors, professional organizations, regulatory/standard-setting organizations and health ministries to help improve vaccine safety and efficacy (5). According to this statement, a particular attention should be paid to the packaging and the labelling of vaccines provided with diluents or of two component vaccines.
- Manufacturers should provide vaccines and liquid components in containers that integrate both components in a single container, such as a dual chambered vial or syringe with powder in one chamber and liquid in the other. The packaging should be assigned based on the component with the nearest expiration date. The packaging should be assigned based on the component with the nearest expiration date.
- Manufacturers should package 2-component vaccines in a way that provides ease of storage and fail-safe preparation of vaccines. For example, if containers that integrate both components in a single container cannot be provided, physically tie the powder and liquid components together. Label each container as container 1 of 2 or 2 of 2.
- All labels of vaccine products that require reconstitution prior to administration should provide clear instructions for mixing vaccine components. The directions for use and a warning to administer the contents of both vials together should be displayed on the front label of the carton, each vial and on the cap of the vial. These recommendations should be prominently hightlighted in summaries of product characteristics and in leaflets. These mandatory documents should also provide information about the required attitude if an error occurred.
- A visual warning should appear on any vaccine diluent or adjuvant liquid provided that indicates it is only a diluent, not the actual product.
- Safety technology such as bar coding or advanced vaccine storage systems should be encouraged by health ministries, product regulators and other authorities. The Global Trade Item Number (GTIN) or national drug code number and associated barcode should appear on each vaccine antigen vial and diluent or adjuvant liquid component.
References:
1. Institute for Safe Medication Practices (ISMP). Administering just the diluent or one of two vaccine components leaves patients unprotected. ISMP Medication Safety Alert!. May 22, 2014; 19 (10): 1-4. Access
2. Al Jazeera. UN: vaccine mistake killed Syrian children. October 1,2014. Access
3. Washington Post. The measles outbreak in Samoa must be a lesson for the rest of the world. December 9, 2019. Access
4. Isaacs, D. Lessons from the tragic measles outbreak in Samoa. Journal of Paediatrics and Child Health 56.1 (2020): 175. Download
5 International Medication Safety Network (IMSN). IMSN Position Statement - Safer design of vaccines packaging and labelling. December 2015: 5 pages. Download
October, 2020
