The International Medication Safety Network (IMSN) is particularly concerned by the risks of error related to antibody-drug conjugates and quickly alerted to these risks.
Beyond the trastuzumab emtansine case, no longer on the WHO INN Programme agenda for a substitution, the IMSN recalls again that other INNs of antibody-drug conjugates fall into the same problem.
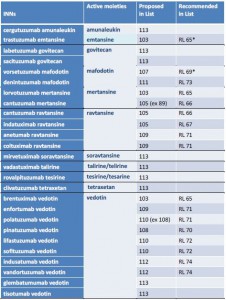 In March 2015, the IMSN called on the WHO INN Programme to change the names of antibody-drug conjugates to reduce their potentially fatal similarities and define clear rules helping to recognize products including different substances and to make them safer.
In March 2015, the IMSN called on the WHO INN Programme to change the names of antibody-drug conjugates to reduce their potentially fatal similarities and define clear rules helping to recognize products including different substances and to make them safer.
In the lack of any risk reduction solutions envisaged by the WHO INN Programme, the IMSN goes further by proposing several approaches in an open letter to the WHO INN Programme on 12 October 2015:
- 1st make awareness on active moieties;
- 2nd change the way of expressing the conjugate compound (taking in account human factor principles, the names of active substances should include a specific prefix for conjugated compound, such as con-, and/or be concatenated by “+”,“plus” or “/” without space);
- 3rd avoid attribution of INN to corresponding naked antibodies when antibody-drug conjugates are considered.
If requested, the IMSN is ready to contribute to the assessment of this risk reduction and prevention strategy, essential but belonging to the sole authority of the WHO INN Programme.
