During the fall of 2020, as the world waited in anticipation for the emergency use/conditional marketing authorization of COVID-19 vaccines, members of the International Medication Safety Network (IMSN) began to discuss safety issues that might impact global immunization roll out efforts with regard to the knowledge on vaccination errors already gathered by the IMSN.

With the goal of sharing experience and learning from member countries to address COVID-19 vaccine safety issues, the IMSN Executive Committee formed the IMSN COVID-19 Vaccine Safety Interest Group (CVSIG) in February 2021. The two primary objectives of the initiative were to address issues encountered by members during global vaccine rollout and to create a guiding document of experience-based safety recommendations.
Building a Response to the Different Types of Vaccine-Related Errors
In a series of CVSIG meetings in mid-2021, as vaccination campaigns rolled out, IMSN members shared firsthand experience with a variety of risks, near-misses, errors, and adverse events following immunization with COVID-19 vaccines on the basis of aggregated analysis by CVSIG members. These included issues with:
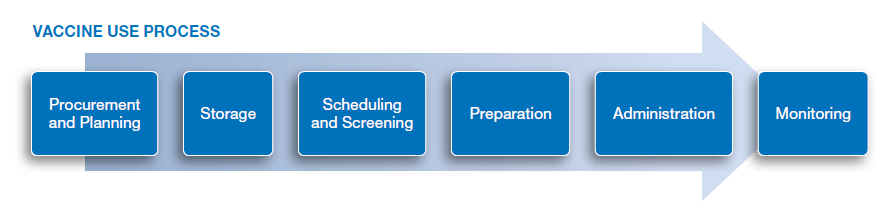
- screening (e.g., wrong age, patients with contraindicated conditions),
- storage (e.g., inappropriate storage temperature, confusion and mis-selection resulting from storage near other vaccines or monoclonal antibodies),
- preparation (e.g., serial errors for vaccines needing a two-step reconstitution process, diluent errors including incorrect diluent volume or no dilution, dosing errors including wrong dose, no dose, expired dose),
- administration (e.g., shoulder injury related to vaccine administration, wrong time interval for second dose or third dose, syringe and/or needle malfunction or misuse leading to underdose, wrong vaccine, wrong drug, wrong route of administration, accidental exposure),
- and monitoring (e.g., missed second or third doses).
In addition, the approach to errors has evolved over the course of COVID-19 vaccination campaigns (for example, intervals between doses, authorized ages, authorized doses, and recommended vaccines).
IMSN CVSIG Recommendations for Global Implementation of Safe COVID-19 Immunization Practices - January 2022
The recommendations follow the vaccine use process and address errors occurring during each stage.

These safety recommendations were developed based on the collective experience and learning from IMSN member countries and should be considered for implementation in global COVID-19 immunization efforts. For a complete copy of the IMSN CVSIG Recommendations for Global Implementation of Safe COVID-19 Immunization Practices, click here.
Moving Forward: during the development of these recommendations, several topics were identified that will receive special attention from IMSN
- Promoting packaging vaccines in prefilled unit-dose syringes as a global standard
- Addressing issues related to COVID-19 vaccine safety for pediatrics as COVID-19 vaccines are being authorized for use in more countries
- Addressing risks during overlapping COVID-19 immunizations and other immunization campaigns
- Involving IMSN in review of a globally standardized schema and pathways for adverse events following immunization reporting
