Intravenous (IV) oxytocin is used antepartum to induce labor in patients with a medical indication, to stimulate or reinforce labor in selected cases of uterine inertia, and as an adjunct in the management of an incomplete, inevitable, or elective abortion. Used postpartum, IV oxytocin is indicated to produce uterine contractions during the expulsion of the placenta, and to prevent or control postpartum bleeding or hemorrhage. Therefore, oxytocin is needed in all obstetric care areas with consistency of practice amongst all provider types (e.g., anesthesia, obstetrics, midwives, perinatal nursing) whether using low doses for labor induction or augmentation, commonly measured in milliunits, or at higher doses to prevent or treat postpartum hemorrhage (PPH), commonly measured in units.
However, improper administration of oxytocin can cause hyperstimulation of the uterus, which in turn can result in fetal distress, the need for an emergency cesarean section, or uterine rupture. Multiple International Medication Safety Network (IMSN) member countries have identified that there are substantial risks involved with the use of oxytocin and have reported significant patient harm from related errors

As a result, the executive committee formed the IMSN Oxytocin Safety Interest Group (OxytocinSIG) to further address the safe use of oxytocin globally. The two primary objectives of the initiative were to share issues and errors on use and administration of oxytocin among members and to develop comprehensive and global applicable recommendations including a guidance document on safe use of oxytocin.
Building a Response to Oxytocin-Related Errors
In a series of OxytocinSIG meetings held in 2022, IMSN members shared firsthand experience with a variety of risks, close calls, errors, and adverse events associated with the use of oxytocin. These included:
- Inappropriate/unnecessary use in labor induction in low-risk patient populations
- Lack of a standardized dosing regimen
- Confusion with look-alike and sound-alike medications
- Inappropriate use of brand names or unsafe abbreviations
- Non-standardized or non-centralized preparation of oxytocin infusions
- Use of multiple oxytocin infusion concentrations/preparations
- Insuficient monitoring of beyond-use dates of pre-prepared solutions
- Reliance on manually programmed infusion pumps without automated safeguards in place
- Mix-ups with infusion tubing
- Mix-ups with dosing/infusion rates
- Use/availability of oxytocin in the direct patient care area without appropriate orders and communication among healthcare providers
IMSN Oxytocin SIG Recommendations for Global Implementation of Safe Oxytocin Use Practices
These selected risk-reduction strategies include recommendations that can help avoid errors and significant patient harm related to the use of oxytocin through all phases of the medication-use process.
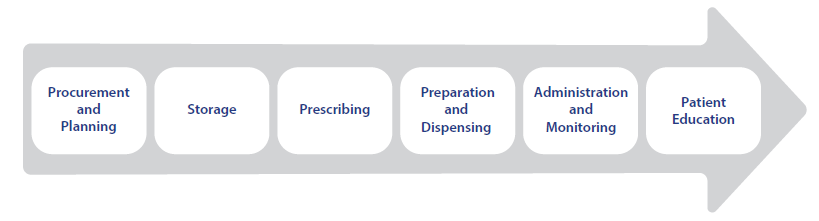
The risk-reduction strategies include high-leverage forcing functions and computerization in addition to less technologically based recommendations. These key improvements must be accompanied by low-leverage strategies, such as staff education, patient education and counseling, and warnings and reminders. Combining low-, moderate-, and high-leverage strategies work to exponentially enhance safe oxytocin utilization.
For a complete copy of the IMSN Oxytocin SIG Recommendations for Global Implementation of Safe Oxytocin Use Practices, click here.

Implementing and moving forward
IMSN acknowledges that healthcare organizations in different parts of the world vary in the availability of technologies (e.g., electronic health records, infusion pumps, automated dispensing cabinets) and resources (e.g., central pharmacy compounding programs). While the recommendations may not all be achievable everywhere, organizations should work to identify those that can be implemented at each step of the medication-use process and consider the remaining recommendations as future goals to work towards.
- Continue to report errors involving oxytocin through your normal reporting programs and to the International Medication Error Reporting Portal, so that we may continue to evaluate the safe use of oxytocin and other medications around the world.
- Premixed solutions are currently not available globally except from outsourced compounding pharmacies in some countries. IMSN urges the global pharmaceutical industry to make premixed oxytocin solutions available everywhere, as soon as possible.
May 2023
